
AmnioBand® Membrane
AmnioBand® Membrane is a minimally processed human allograft which retains the structural properties of the extracellular matrix. The resulting dehydrated allograft serves as a wound covering.
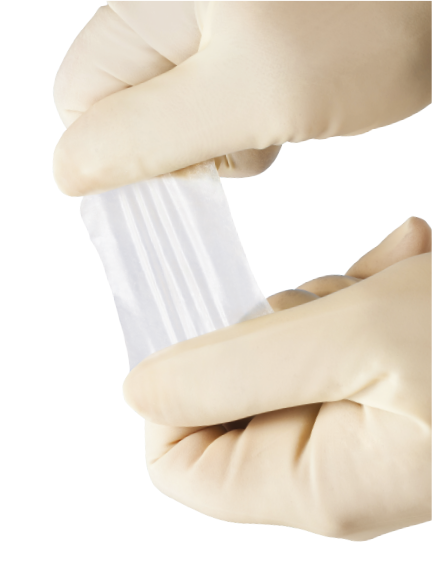
Key Features:
-
- MTF Biologics’ proprietary aseptic processing method maintains the inherent growth factors and matrix proteins native to the tissue; all of which are known in literature for their roles within the natural closure of wounds
- Aseptic processing preserves tissue’s natural structure
- Ready, right out of the package
- Can be used in the hydrated or dehydrated state
<li “> Available in multiple sizes
- Shelf life of three years at ambient temperature
- Flexible
- Conforms to anatomy and maintains surface contact
| Order No. | Product Specifications | UPC |
|---|---|---|
| WC3010 | AmnioBand Membrane, 10mm Disk | 840045712847 |
| WC3014 | AmnioBand Membrane, 14mm Disk | 840045712854 |
| WC3016 | AmnioBand Membrane, 16mm Disk | 840045712861 |
| WC3018 | AmnioBand Membrane, 18mm Disk | 840045712878 |
| WC3022 | AmnioBand Membrane, 2cm x 2cm | 840045712885 |
| WC3023 | AmnioBand Membrane, 2cm x 3cm | 840045712892 |
| WC3024 | AmnioBand Membrane, 2cm x 4cm | 840045712908 |
| WC3034 | AmnioBand Membrane, 3cm x 4cm | 840045712915 |
| WC3044 | AmnioBand Membrane, 4cm x 4cm | 840045712939 |
| WC3038 | AmnioBand Membrane, 3cm x 8cm | 840045712922 |
| WC3046 | AmnioBand Membrane, 4cm x 6cm | 840045712946 |
| WC3056 | AmnioBand Membrane, 5cm x 6cm | 840045712953 |
| WC3077 | AmnioBand Membrane, 7cm x 7cm | 840045712960 |
OUR TEAM
Behind our innovative wound care solutions is a dedicated team, passionate about advancing healing and enhancing patient outcomes. Committed to excellence, we work hand-in-hand with clinicians to deliver superior care.

ANTHONY KIRCHNER
Founder & Ceo

DOMINIQUE ARANGO
Midwest Sales Manager

MIRLENE DENOYE
Director of Sales

JOHN GARRETTSON
Chief Strategy Officer



